-
Make a Call : +86 551 63500087
-
Get A Estimate : sales@gnfchem.com
What Are You Looking For?
What Are You Looking For?
Make a Call : +86 551 63500087
Get A Estimate : sales@gnfchem.com

Why is vinegar sour?
Aug 07, 2024Vinegar is a traditional condiment in all major Chinese cuisines. The sour flavour can give people a refreshing and stimulating feeling, and has the effect of enhancing appetite. According to the existing text, the ancient Chinese working people used wine as a fermentation agent to ferment and brew vinegar, vinegar originated in China, according to the literature, the history of vinegar brewing reaches more than 3,000 years.
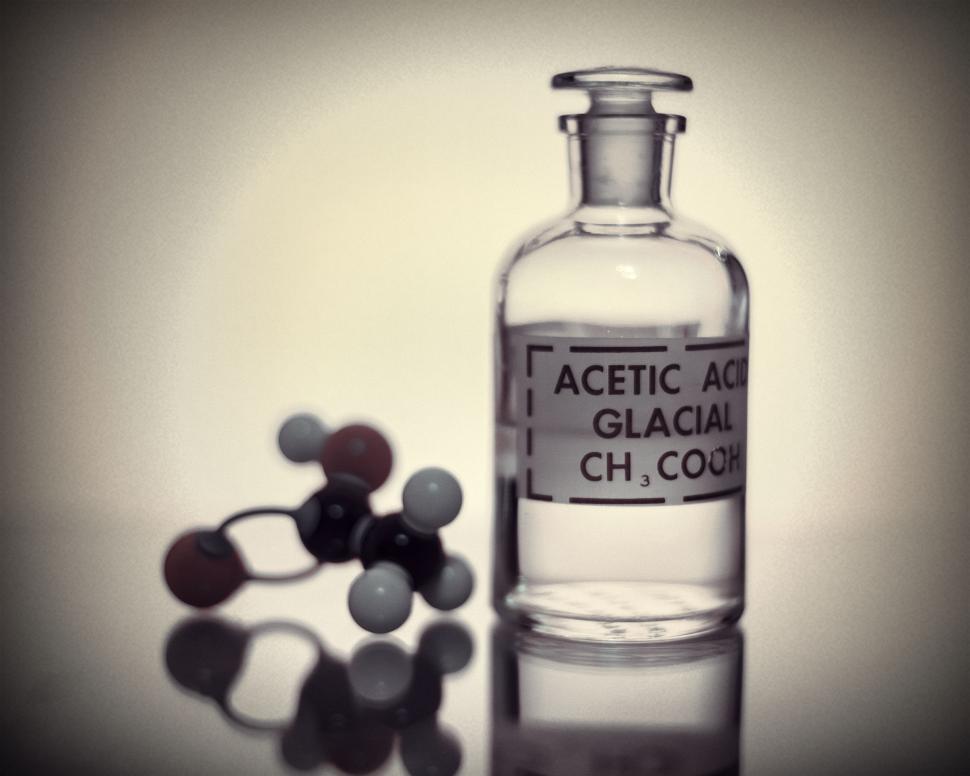
Vinegar is sour mainly because it contains acetic acid. The chemical formula is CH3COOH, which is a kind of organic monobasic acid, and the aqueous solution is weakly acidic. In aqueous solution, due to the role of water molecules, acetic acid will be partially dissociated into hydrogen ions (H +) and acetate ions (CH3COO-): CH3COOH H + + CH3COO-.
Why is the presence of acetic acid an acid? Because the sour taste is the characteristic taste of substances such as inorganic acids, organic acids and their acidic salts. The substance itself that has a sour taste is hydrogen ions.
Studies have shown that there are three possible ways in which hydrogen ions can stimulate taste cells: by entering the taste cells through ion channels or free diffusion; by preventing potassium ions from passing through the ion channels; by binding to the ion channels, which opens the channels and allows other cations to pass through the channels into the taste cells; and, finally, by altering the electrical potential of the cell membranes, which induces neural signals to be transmitted. However, scientists have not yet come to a definitive conclusion as to which of these modalities or which of these modalities work together.
There is not a simple correlation between the strength of the sour taste and the concentration of the acid. The different sour tastes that various acidulants have and the sour sensation that they cause in the mouth are related to the type of acid root, the pH, and the presence of other substances, especially sugars. At the same pH, organic acids are more acidic than inorganic acids. However, at the same concentration, the intensity of the acidity of various acids varies, mainly due to the effect of the dissociated anions of the acidulant on the sense of taste. Therefore, the acidity of one acid cannot completely replace the acidity of another acid by an equal weight or concentration. Comparing the intensity of acidity of different acids at the same concentration, the order is: hydrochloric acid > nitric acid > sulphuric acid > formic acid > acetic acid > citric acid > malic acid > lactic acid > butyric acid.
In addition, the human perception of acidity increases with temperature. On the one hand, because the ionisation degree of acid increases with temperature; on the other hand, because some buffers such as proteins are denatured after the temperature rises, thus losing their buffering effect, resulting in the enhancement of sour taste.
Generally in the same concentration of ionisation degree of acid, because of its high hydrogen ion concentration, so the acidic flavour performance is also stronger. Food and beverage processing in the use of organic acids more types of organic acids, these organic acids taste different, with different anion composition. When several organic acids are used together, there is no enhancement of the sour taste, but it can adjust the taste quality, so that it has a certain characteristic.
There is also an attenuating effect between acidity and other flavours. Sweet substances in a small amount of acid, the sweetness is weakened, sweet substances in the acid is weakened sourness. Adding a small amount of salt to a sour flavour weakens the sourness, but adding a small amount of acid to salt enhances the saltiness. Adding a small amount of salt to pomelo reduces the sourness and accentuates the sweetness when it is eaten. A small amount of bitter or astringent substances in the acid will enhance the sourness. In the case of fruits such as lemon and grapefruit, the right amount of bitterness can be combined with acidity to bring out the flavour.
In addition to acetic acid, citric acid is also a common acidulant, and citric acid is the most widely used. Now mostly prepared by fermentation method. Citric acid is colourless translucent crystals or white particles or white crystalline powder, with a rounded and refreshing sour taste. Citric acid is particularly suitable for citrus beverages and is also used alone or in combination in other beverages.
GNFCHEM is professional Food Additives manufacturer, please follows us and get more products catalogue and price!
Hi! Click one of our members below to chat on